Constancy of the optimum temperature of an enzyme under varying concentrations of substrate and of enzyme / by Arthur Compton.
- Compton, Arthur.
- Date:
- 1914
Licence: In copyright
Credit: Constancy of the optimum temperature of an enzyme under varying concentrations of substrate and of enzyme / by Arthur Compton. Source: Wellcome Collection.
Provider: This material has been provided by The Royal College of Surgeons of England. The original may be consulted at The Royal College of Surgeons of England.
4/8 (page 259)
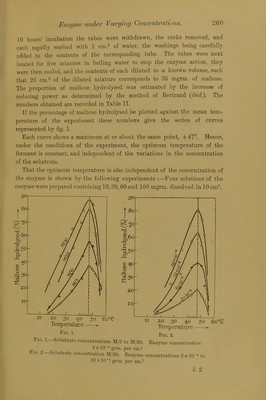
![Mr. A. Compton. Optimum Temperature of an In the present paper further experimental evidence for this hypothesis is given, in the case of another hydrolytic enzyme, the maltase of Aspergillus oryzce (taka-diastase). For the extract of Aspergillus oryzce used, the Imperial Cancer Eesearch Fund is indebted to Messrs. Parke, Davis and Co., who placed at my disposal one of their most active preparations. This preparation, after being freed from insoluble constituents and purified by a technique to be detailed elsewhere, consists of a white powder, entirely soluble in water, whose activity in maltose is double that of the original preparation. The maltose used was Kahlbaum’s. It was purified by successive recrystallisations from water, the mother liquor impurities being removed after each recrystallisation by pressing the crystals in an hydraulic press between several layers of clean dry linen. Eventually, after powdering in a mortar and drying for about a week in vacuo over sulphuric acid, a specimen of pure maltose, containing one molecule of water of crystallisation, was obtained. It gave an optical activity [«]•*> = + 130-4°, and its reducing power, determined by Bertrand’s method,* was as set out in Table I. Table I. Weight of maltose. Weight of copper. mgrm. mgrm. 20 -0 21 -0 40 -0 42'0 60 -0 62 -0 80 -0 83 -0 100 -o 103 -5 These numbers, allowing for the molecule of water of crystallisation present, correspond exactly with those given by Bertrand (ibid.). That the optimum temperature of the ferment is independent of the concentration of the substrate is shown by the following experiments :—Four series of eight clean Jena glass test-tubes were prepared containing respec- tively 360, 180, 90, and 60 mgrm. of maltose dissolved in 4 cm.3 of water which had been specially purified by redistillation under diminished pressure. Then into each tube was introduced in portions of 1 cm.3 a solution of the enzyme, prepared a half to one hour previously, containing 10 mgrm. per cm.3. The substrate concentrations in the four series of tubes are M/5, M/10, M/20, and M/30. The tubes, after being closed with clean sterile corks, were plunged into water-baths kept at known temperatures. After * ‘Bull. Soc. Cliim.,’ (3), vol. 35, p. 1285 (1906).](https://iiif.wellcomecollection.org/image/b22447829_0006.jp2/full/800%2C/0/default.jpg)